One of the many problems with the pharma regulation debate in India is the tendency to focus more on “spurious drugs” rather than “sub-standard drugs”. Chances are, we hear more about “Spurious Drugs” when both practitioners (doctors) and patients talk about the quality of our drug supply. It is rare that we ever hear of substandard drugs. Why is that?
“Spurious drugs” which are the same as counterfeit drugs are manufactured with the sole intent of financial gain by passing of fakes as a legitimate product. On the other hand, substandard drugs are manufactured by legitimate manufacturers under their own name but without following good manufacturing practices. As a result, these products are either lower in potency compared to what they say on the label, or contain other substances which are harmful to the patients who take them. It is necessary to draw a distinction between these two because spurious drugs are a ‘law & order’ problem while substandard drugs are a regulatory problem. Contrary to the general belief, substandard drugs are a much bigger problem than spurious or counterfeit drugs in India. The figures below provide hard evidence of this trend:
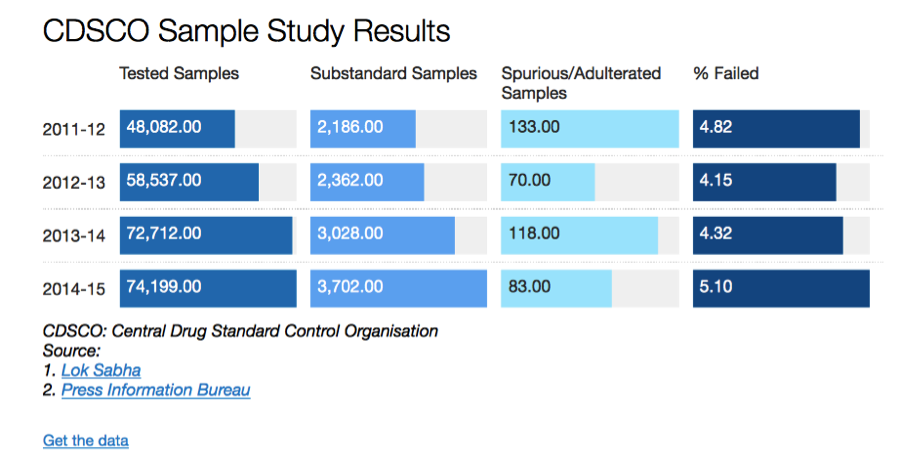
Source: http://www.indiaspend.com/cover-story/1-in-7-indian-drugs-revealed-as-substandard-97614
There is a rather simple rationale for this trend. Since the intent behind counterfeiting is financial gain, it makes sense for the counterfeiters to go after high priced products. For example, it makes sense to produce a counterfeit Prada or Louis Vuitton handbags because of the brand equity (price) they command in the market. It makes no sense to counterfeit a no-name product, because the financial incentive simply doesn’t exist. In a country like India, where price-controls are the norm in the pharmaceutical industry; and even for those medicines that are not under the purview of government imposed price controls where the price-point is extremely competitive, does it really make sense for anyone to produce a counterfeit drug? The answer is evident in the chart above.
As you can see, we don’t really have a problem with Spurious Drugs; we do indeed have a problem with Substandard Drugs. The data we have collected through the RTI process tells us that while these numbers are severely under-reported, the trend holds. The prevalence of spurious drugs is infinitesimally small compared to the prevalence of substandard drugs. So why is it that the public debate in India focuses more on spurious drugs?
Our research indicates several reasons for this. One of the likely reasons for this confusion is that there is the generally poor level of awareness about drug regulation in India. There is little understanding about the difference between what is a spurious drug and what is substandard. If we expect grassroot level awareness about the problem with our drug supply, lets first make sure we get the nomenclature right. A less likely, but equally culpable reason is the confusing terminology intentionally used in the Drugs & Cosmetics Act, 1940 to deal with various categories of defective drugs. This is an issue which requires a more detailed explanation.
The various definitions in the Drugs & Cosmetics Act
The law currently contains four different categories of “offences” with respect to quality of a drug or its packaging: “misbranded drugs”, “spurious drugs”, “adulterated drugs” and drugs which fail to comply with “standards of quality” laid down in the Second Schedule to the Drugs & Cosmetics Act, 1940.
Broadly speaking, “misbranded” covers drugs which aren’t labelled as per the law or if the drug makes a false claim for any of its ingredients or if a drug is coloured or powdered to conceal damage and misrepresent its therapeutic value. “Spurious drugs” covers the offence of misrepresenting the manufacturer of the drug or misrepresenting the active ingredient of the drug itself. “Adulterated drugs” includes the addition of any substance that is filthy or putrid or reduces the quality or strength of the drug or if it is prepared and packed in insanitary conditions. Interestingly, “Sub-standard” is not defined in the Act; instead Section 18(a)(1) merely states that the manufacture of drugs which are not of standard quality is prohibited. So much for downplaying the real problem with our drug supply. Mind you, all of the above definitions have been simplified for the sake of this blog post since the actual definitions are a lot more complicated, confusing and overlapping. Various components of the definitions of “misbranded” and “adulterated”, are actually covered by the definition of “not of standard quality”. The graphic below depicts how poorly these categories are defined.
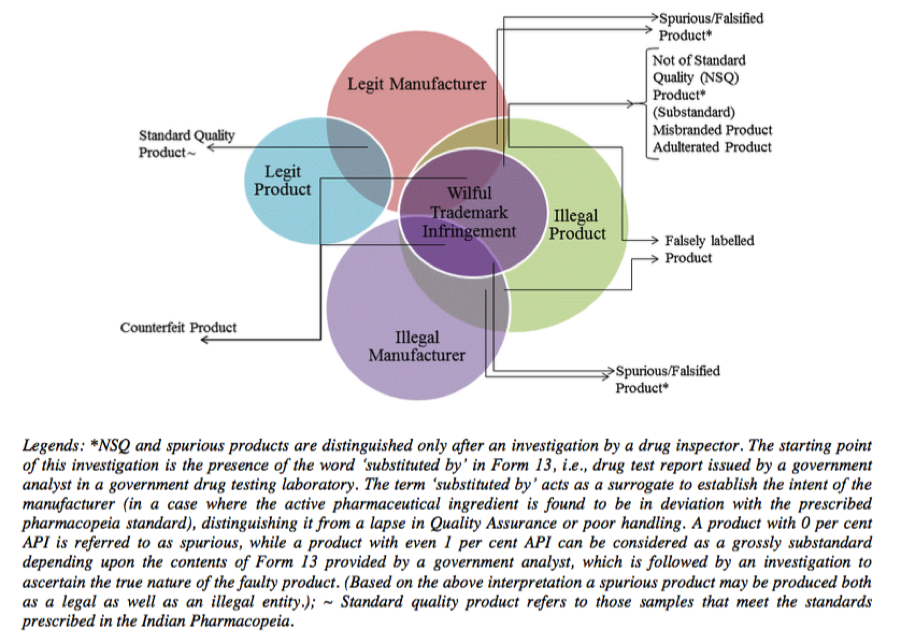
Source: http://icrier.org/pdf/Working_Paper_310.pdf
As if the confusion created by these arbitrary classification is not enough, there are the DCC guidelines discussed in an earlier post, which create artificial categories like “grossly sub-standard drugs” and drugs with “minor defects”. Does it come as a surprise to anyone that the most common parlance for public discussion therefore gravitates toward the terminology most “marketed” by the industry, namely Spurious drugs?
The multiple definitions of, what is essentially bad medicine, directly affects the legal and public debate because it creates confusion in the minds of policy-makers and journalists as to which type of offence covers what kind of defect in the drug. This is perhaps one of the reasons we see people in public office and media use spurious, adulterated, misbranded and not of standard quality in an interchangeable fashion.
The American approach to defining bad medicine
On the other hand, the United States for example has two simple categories: one is adulterated drugs and the other is misbranded drugs. Although the definitions are detailed, the simple categorisation helps to simplify the public debate since the media has to deal with only two simple categories.
An additional advantage of the American approach over the Indian approach is that it focuses even on manufacturing processes unlike the Indian definitions which focus only on the end product. Under American law, there is a presumption that if a drug fails to comply with good manufacturing practices (cGMP), the end product is adulterated. I reproduce the relevant portion of the definition: “if it is a drug and the methods used in, or the facilities or controls used for, its manufacture, processing, packing, or holding do not conform to or are not operated or administered in conformity with current good manufacturing practice to assure that such drug meets the requirements of this chapter as to safety and has the identity and strength, and meets the quality and purity characteristics, which it purports or is represented to possess”
There is no such presumption in Indian law. Our approach to quality is focussed on evaluating the marketed drug against a set of standards established by the Indian Pharmacopoeia. No wonder that while the American and European drug inspectors repeatedly find and penalize questionable practices among the Indian manufacturers, the CDSCO’s track record is all but making empty threats.
This is one of the reasons why one of my PILs sought to clean the current slate so that we could begin by creating a modern framework for regulating drugs in India. Unfortunately, the Supreme Court thought this was an academic issue. The fact that such confusion affects law enforcement and public health of over a billion people who live in India is somehow lost in translation.

I guess folks who produce aduletrated drugs do not care. Global average death rate is about 8 per 1,000. If the rate goes up by two per 10,ooo, it does not matter much as they will never be caught. So why worry.
It is ironic that folks call themselves God fearing but when it comes to making money everything is kosher.
Very good article. I certainly appreciate this website.
Continue the good work!