In addition to the process of criminal prosecution to punish violators of the D&C Act, the Drugs & Cosmetics Rules, 1945 also provide for suspending or cancelling the manufacturing licences of the company found to have manufactured NSQ drug. As explained in an earlier post on DCC Guidelines for Prosecutions, drug inspectors have been advised to opt for suspension/cancellation of licences in most NSQ cases, rather than opt for criminal prosecution. Given that suspensions/cancellations are the preferred mode of enforcement in India, its necessary to understand how this system actually works in practice.
The relevant rule in this regard is Rule 85(2) of the Drugs & Cosmetics Rules, 1945.
(2) The Licensing Authority may, for such licences granted or renewed by him, after giving the licensee an opportunity to show cause why such an order should not be passed, by an order in writing stating the reason therefor, cancel a licence issued under this Part or suspend it for such period as he thinks fit, either wholly or in respect of some of the substances to which it relates, [or direct the licensee to stop manufacture, sale or distribution of the said drugs and an Inspector] if, in his opinion, the licensee has failed to comply with any of the conditions of the licence or with any provision of the Act or Rules thereunder.
This power is exercised by Drug Controllers in individual states, since it is State Governments and not the Central Government which licences drug manufacturing in India. This Rule states that the licensing authority may, after giving the licensee an opportunity to show cause, cancel a licence issued or suspend it for such period as he thinks fit either for the facility or for manufacture of a specific drug.
The problem with this provision is that since each State Licensing Authority (SLA) operates independently of the others, there is no uniformity in the duration for which licences are suspended or cancelled. We have near certain information of this practice from copies of the Register of NSQ drugs maintained by the Karnataka Drugs Control Department (KDCD) that we procured under the Right to Information Act, 2005. This Register contains details of all the NSQ drugs detected by the KDCD within the state of Karnataka and the action taken against them by their respective drug controllers. Since a majority of the NSQ drugs were actually being manufactured outside the state, the KDCD did not have the power to suspend or cancel licences for most of these manufacturers. In such cases, the Drug Inspectors from Karnataka would write to their counterparts in the other states and request them for action to be taken against the manufacturers located in those states. The drug inspector in the home state of the manufacturer would then write back to the Karnataka Drug Inspector informing them of any action taken against the manufacturer in terms of either suspension or cancellation of licensees. The action taken by these other SLAs would be jotted down in the register in a hand-written format.
Below is a graphical representation of the states from which the KDCD detected NSQ drugs in the year 2012-13.
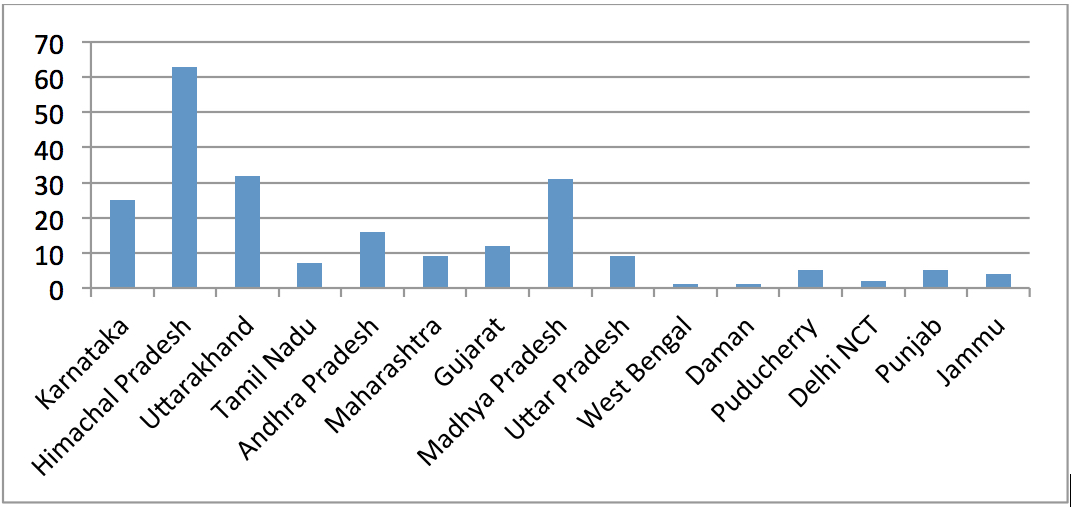
Here is another graphical representation of the states from which the KDCD detected NSQ drugs in the year 2011-12.
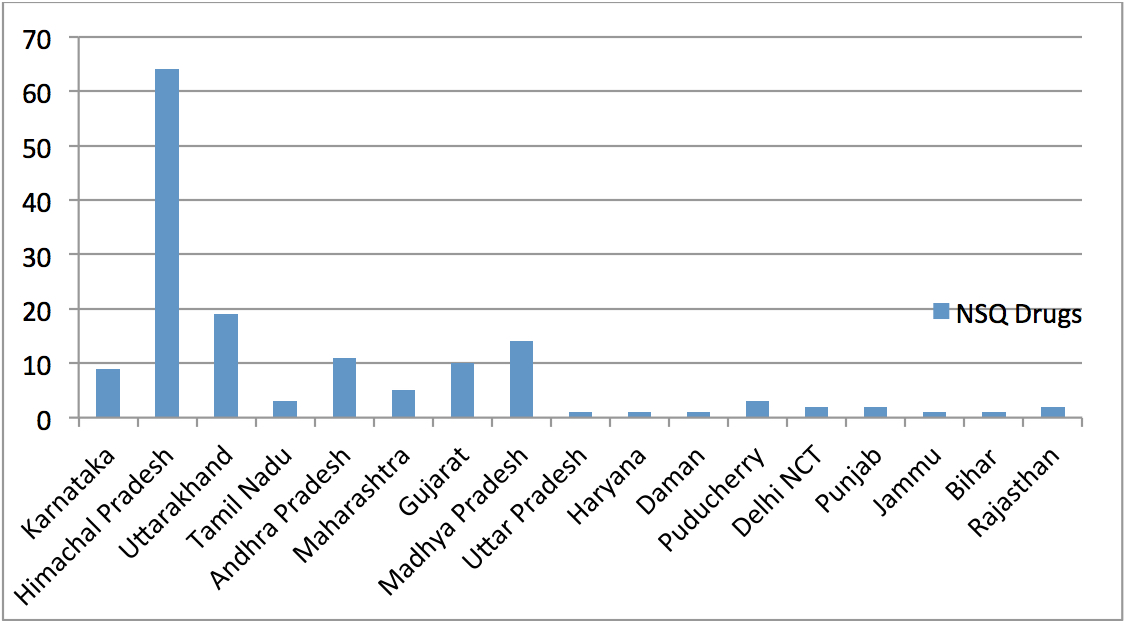
The two states accounting for the largest number of manufacturers of NSQ drugs every year in Karnataka are Himachal Pradesh and Uttarakhand, with Madhya Pradesh coming a close third. From the details contained in the Registers, it is quite obvious that there is no consistency amongst different states in the manner in which licences of erring manufacturers are suspended. For example, while states like Himachal Pradesh suspend licences from anywhere between 15 days to 3 months, states like Uttarakhand would suspend licences for a mere 20 days while a state like Gujarat would suspend licence for just 1 day. This large scale discrepancy in the duration for which licences are suspended in different states is because there are no rules notified by the Ministry of Health and Family Welfare under the D&C Act requiring all SLAs to follow uniform standards while suspending licences. Thus each SLA appears to exercise its own discretion while suspending licences.
A second and more serious issue with the practice of suspending licences is whether SLAs actually enforce their orders suspending manufacturing licences. Typically if these suspension orders were being aggressively enforced, one would expect to find several cases in the High Courts by pharmaceutical companies challenging the suspension orders and seeking stay orders. However a search of the reported judgments of the High Court for the state of Himachal Pradesh (the biggest source of NSQ drugs) did not reveal a single case where the issue of a suspended licence was challenged in the High Court. Given how combative the industry is when it comes to any punitive action affecting their profits as we see from the response to the FDC ban, it’s strange that none of the suspensions have ever been challenged before the High Courts. This calls into question how effectively are the SLAs enforcing their own orders of holding these errant manufacturers accountable for their action in flooding the market with substandard drugs.
